The ISO/IEC 17025 standard is a management system standard which specifies the general requirements for the competence to carry out tests and/or calibrations, including sampling. It covers testing and calibration performed using standard methods, non-standard methods, and laboratory-developed methods. It is applicable to all organizations performing tests and/or calibrations. These include, for example, first-, second- and third-party laboratories, and laboratories where testing and/or calibration forms part of inspection and product certification.
Since the focus of ISO 17025 is on the operation and management of the laboratory, it comes as no surprise that Laboratory Information Management Systems (LIMS) can have an important role to play. However, a well configured LIMS, such as Matrix Gemini can offer much more than data management surrounding sampling, testing and reporting. It can make a significant contribution to the inherent processes that underpin ISO 17025. This is the first in a series of articles which will look at specific areas of Matrix Gemini and see how they relate to the requirements of ISO 17025.
Managing documents
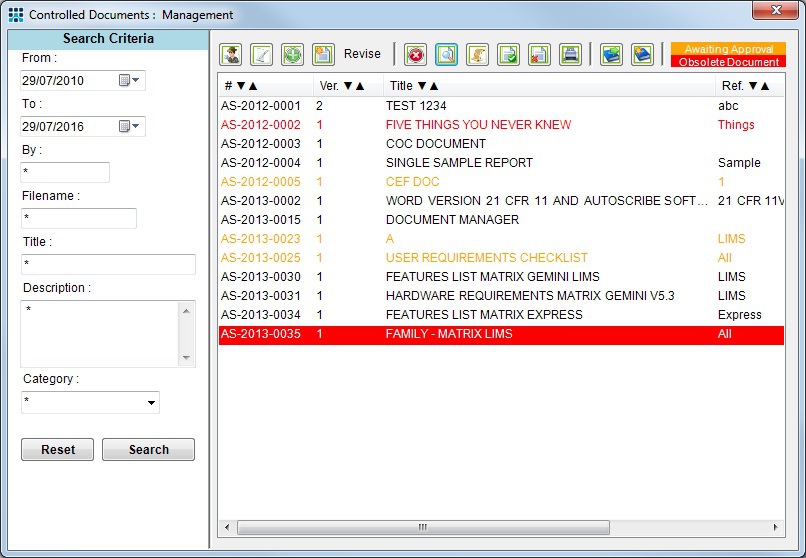
All of the process and procedures established as part of ISO 17025 need to be documented and provision made for access for everyone who needs them. Issues such as the appropriate indexing and monitoring of document updates also need to be addressed. Matrix Gemini Document Manager is one of the modules in the Matrix Quality Management Suite. It can track, store and distribute all documents from a central location. Version control provides a full audit trail allowing the history of revised documents to be traced for internal and external auditing and reporting. Documents can never be lost as saved information is automatically backed up. Staff can be asked to record the fact that they have read, understood and signed-off controlled documents and reports can be generated to show staff have read appropriate documents for the purposes of ISO 17025.
Example Document Management Screen showing version control of newly released documents
Applying Document Manager to ISO 17025
We can take a look at some of the specific areas within ISO 17025 where Document Manager can meet the requirements. Section 4 covers all the management requirements for the standard, while Section 5 addresses the technical requirements. There are numerous sub sections where Document Manager is relevant.
Part of Section 4.2.1 requires that the system’s documentation shall be communicated to and available to the appropriate personnel. Section 4.3 requires the laboratory to establish and maintain procedures to control all internal and external documents that form part of its management system. From Section 4.3.2.1 comes the need to establish a master list or an equivalent document control procedure identifying the current revision status and distribution of documents in the management system to preclude the use of invalid and/or obsolete documents. Document Manager also fulfills the requirement of Section 4.3.3.4 to have a procedure that describes how changes in computerized documents maintained are made and controlled.
From Section 4.13.1.1 laboratories must establish and maintain procedures for indexing, access, filing, storage, maintenance and disposal of quality and technical records. Moving to the technical requirements, Section 5.4.1 specifies that all instructions, standards, manuals and reference data relevant to the work of the laboratory must be kept up to date and made readily available to personnel (see 4.3). Any accepted deviation from a test and calibration method must be documented. Finally, in Section 5.5.5 there is a requirement to maintain records of each item of equipment and its software significant to the tests and/or calibrations performed.
The Matrix Gemini Document Manager can fulfill of the requirements outlined above. In the next article in this series, we will look at how Matrix Gemini handles Corrective and Preventative Action (CAPA) requirements. A copy of ISO 17025 can be purchased online at: www.iso.org/obp/ui/#iso:std:is…
If you can’t wait for the next instalment call the experts on +44 118 984 0610 to find out how Matrix Gemini could help your lab meet ISO17025 requirements.
To find out more visit: https://www.autoscribeinformatics.com/